Like many, I waited with bated breath for results of the anti-PCSK9 (evolocumab) FOURIER cardiovascular outcome study last week. There have been many interesting commentaries written on the findings. A few of my favorites are listed here (Matthew Herper), here (David Grainger), here (Derek Lowe), and here (Larry Husten), amongst others, with summaries provided at the end of this blog. Most of these articles focused on clinical risk reduction vs. what was predicted for cardiovascular outcome, as well as whether payers will cover the cost of the drugs. These are incredibly important topics, and I won’t comment on them further here, other than to say that the debate is now about who should get the drug and how much it should cost.
In this blog, I want to emphasize key points that pertain to human genetics and drug discovery. And make no mistake: the anti-PCSK9 story and FOURIER clinical trial outcome is a triumph for genetics and drug discovery. This message seems to be getting muddled, however, given the current cost of evolocumab and the observation that cardiovascular risk reduction was less than expected, based on predictions from a 2005 study published by Cholesterol Treatment Trialists (CTT) (see Lancet study here). [Actually, this statement is not entirely accurate, as the 15% risk reduction used in FOURIER is a composite endpoint of cardiovascular (CV) death, myocardial infarction (MI), stroke, coronary revascularization, or hospitalization for unstable angina. The CTT-line is based on CV death, MI, stroke, or coronary revascularization, but not unstable angina. If FOURIER outcomes are limited to heart attacks – which has been the primary genetic link with PCSK9 variants – then risk reduction is 27%, or close to that predicted by the CTT-line.]
The key point here is that I continue to believe that the anti-PCSK9 / FOURIER story provides proof-of-concept for the future of genetic-driven drug discovery. This is no one hit wonder; this is not the “My Sharona” or “Tainted Love” of drug discovery & development. The anti-PCSK9 story reinforces concepts that human genetics can (1) identify novel drug targets; (2) speed pre-clinical development; (3) provide confidence in surrogate endpoints for regulatory approval; and (4) guide dose-response estimates, and therefore target dose for a large and expensive clinical trial.
This does not mean that routine implementation of genetic-drive drug discovery is nigh. Alas, routine implementation will take time (see my recent blog here). But I do think it is worth reflecting on key points of the anti-PCSK9 story leading up to FOURIER, and put these points in context of broader drug-discovery.
(1) Discovering unexpected drug targets. Before the first PCSK9 genetic study was published in 2003 (see here), there was only a single PubMed article published on PCSK9…and that was in the same year by the same group (see here). In other words, nothing was known about PCSK9 before a variant piece of DNA was shown to track with clinical outcomes in a large family. Shortly after the seminal discovery that a gain-of-function mutation raises LDL levels, loss-of-function variants were identified that lowered LDL cholesterol levels (here) and protected from cardiovascular disease (here). Together, these findings provided direct genetic evidence that gain- and loss-of-function mutations in PCSK9 are medically relevant, and therefore that PCSK9 is an attractive drug target.
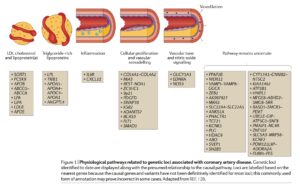
What is remarkable is that most of the genes identified from human genetics in heart disease cannot be ascribed to known biological pathways (see recent review by Khera and Kathiresan here, figure below). Moreover, even for those genes that can be ascribed to pathways such as inflammation, vascular tone, and cellular proliferation / vascular remodeling, the protein products of those disease-associated genes have not yet been successfully targeted with therapeutic molecules. This raises the intriguing possibility that there are many novel drug targets within the list of disease-associated cardiovascular genes, not to mention the many genes that remain to be discovered.
It may seem obvious that biopharma needs to identify novel drug targets that differentiate from standard of care therapy. After all, there is increasing pressure from regulatory authorities to approve, and payers to pay for, therapies that are unambiguously different from what is available to patients today. However, the scientific tools available to find truly novel targets that differentiate from standard of care are not easy to come by, especially if one relies on targets with a clear link to causal human biology. Human genetics represents one such approach to novel targets.
Big picture: Even though PCSK9 acts through well-established LDL cholesterol pathways for reducing risk of cardiovascular disease, FOURIER provides proof-of-concept that human genetics can discover unexpected and novel targets that differentiate from standard of care therapies (e.g., statins).
(2) Timeline from target discovery to clinical proof-of-concept. It is often cited that a it takes 8-10 years to take an idea and show that it works in humans for the first time. The reality is it takes much longer, as many years of fundamental basic research – often funded by the NIH and done by academics – are required to understand the biology of a putative therapeutic target.
For PCSK9, the unknown biology was unraveled with remarkable speed. A timeline for one of the two anti-PCSK9 monoclonal antibodies, alicrocumab (Praluent), is shown in the figure below. A key scientific advance was the recognition that PCSK9 is secreted from cells and interacts with the LDL receptor on the surface of cells (reviewed here). This observation made PCSK9 targetable with a monoclonal antibody (which can bind to proteins outside of cells) rather than a small molecule (which often work by inhibiting proteins inside of cells).
Once the biology of genetic targets is understood sufficiently to generate a therapeutic hypothesis, this information guides the use of pre-clinical models to test pharmacology and safety and thus shorten the timeline to clinical proof-on-concept. That is, targets rooted in causal human biology have human validation; pre-clinical models are not necessary for efficacy per se (e.g., cardiovascular protection). The purpose of pre-clinical models, therefore, is not to establish evidence of efficacy, but to ensure that the therapeutic molecule is recapitulating human biology and is safe to test in humans. For anti-PCSK9 therapy, pre-clinical animal models were applied to confirm that monoclonal antibodies would lower LDL levels and be synergistic with statin therapy (here). This led to confidence and an expedited path to human clinical trials.
Unfortunately, the timeline from gene discovery to clinical trial is much slower for most genetic targets. This represents the current bottleneck for genetic-driven drug discovery and development. Cystic fibrosis (see YouTube video by David Altshuler, CSO Vertex here) and sickle cell anemia (recent NEJM paper by Bluebird bio here, my blog here) represent two such examples – it took decades from gene discovery to therapeutic invention. Nonetheless, once the biology is understand in sufficient detail to formulate a sound therapeutic hypothesis, pre-clinical development can occur quite quickly, which speeds the time to a human clinical trial.
Big picture: The timeline from target discovery to proof-of-concept in humans was remarkably fast for anti-PCSK9 therapy. Thus, the anti-PCSK9 story offers hope that drug discovery for other genetic targets will speed rapidly through clinical development and demonstration of clinical benefit, once the biology of those targets are also understood.
(3) Applying biomarkers as surrogate endpoints for FDA approval. With the appointment of Dr. Scott Gottlieb as FDA Commissioner, there has been increased talk of using surrogate endpoints for FDA approval (see Endpoints story by John Carroll here). In truth, this has been an active topic for sometime. [Clinical endpoints measure how a patient feels or functions, or whether a patient lives longer. In contrast, surrogate endpoints are biomarkers, such as a laboratory test, radiographic image, or physical signs (e.g., blood pressure) that substitute for clinical endpoints.]
According to the FDA website, approximately 45% of drugs approved by the FDA between 2010-2014 were approved with surrogate endpoints. Similarly, a study in JAMA by Yale cardiologist Harlan Krumholz and colleagues found that 45% of drugs approved between 2005-2012 were approved with surrogate endpoints (see here). Many of these endpoints, however, are clinical in nature (e.g., progression free survival in cancer) or biomarkers used routinely for decision-making in clinical care (e.g., HgbA1C for diabetes, FEV1 for asthma or COPD, viral load in HIV). There is certainly an opportunity to expand surrogate endpoints into those biomarkers that are not part of routine clinical care.
The PCSK9 story provides one of the best examples of a surrogate endpoint for FDA approval, albeit one the is deeply established in patient care. Today, physicians use LDL cholesterol to predict risk of heart disease and target therapy to lower LDL levels based on risk profile. In addition, human genetics and a technique known as Mendelian randomization has established that LDL is causally related to cardiovascular disease. Accordingly, the FDA allowed approval of anti-PCSK9 therapies with the agreement that post-approval cardiovascular outcome studies would be conducted.
My prediction is that as tools such as Mendelian randomization become more established, and as targets are selected based on human genetics, then surrogate endpoints will become even more powerful and effective at predicting clinical outcomes. Moreover, there will be greater comfort using biomarkers not used routinely in clinical care as surrogate endpoints for approval. Such confidence will speed the path from early proof-of-concept clinical trials to regulatory approval.
Towards this end, resources such as large-scale biobanks (e.g., see here the recent GSK/Regeneron news to exome sequence 500,000 individuals in the UK Biobank) and protein quantitative trait loci (pQTL) measurements (see recent Nature Communications article here) will create new opportunities for surrogate biomarkers in regulatory approval.
There is a note of caution, however, as a recent study published in JAHA (see here) concluded that nearly half of the positive surrogate trials were not validated in clinical outcome studies. This study is a reminder that much work remains to establish meaningful surrogate endpoints.
Big picture: Anti-PCSK9 therapy provides a compelling example of a biomarker (LDL) as surrogate endpoint to expedite regulatory approval. FOURIER, which represents the cardiovascular outcome trial that was conducted post-approval to demonstrate clinical efficacy, increases confidence that biomarkers based on human genetics and Mendelian randomization will expedite drug development.
(4) Demonstrating dose-response curves based on human genetics. What is the optimal dose for a large, expensive clinical trial such are FOURIER? How low is low enough for LDL-lowering therapies? FOURIER, which included 27,564 patients with cardiovascular disease and LDL levels above 70 mg/dl already receiving statins, lowered LDL levels to an astonishing 30 mg/dl. By why 30…and not 50 or 10 mg/dl?
Human genetics helped guide this choice of LDL goal.
The balance between safety and efficacy is what drives the choice of dose and duration for every drug trial. The original disease-associated LoF variants described by Helen Hobbs and Jonathan Cohen were present in a heterozygous state. A subsequent study found an individual who inherited two inactivating mutations in PCSK9 and had a strikingly low plasma level of LDL-C (14 mg/dL), but was otherwise healthy (here). That is, a precious single individual who had no detectable PCSK9 was just fine, indicating that it should be safe to drive down LDL levels <30 via a PCSK9-mediated mechanism.
What is incredibly exciting is that there are now broad initiatives to catalogue “human knockouts” at an unprecedented scale (see East London Genes & Health, PROMISE cohort as examples). If clinical data for such human knockouts are available, and if individuals have been consented for recall to allow additional phenotyping, then it should be possible to estimate the clinical impact of perturbing targets via human genetics. (For a deeper discussion on this topic, see Nature Reviews Drug Discovery article here.)
Big picture: Human genetics predicted the “maximum tolerated dose” that would also be effective in protecting from cardiovascular events in the FOURIER trial. As catalogues of human knockouts become more available, this approach should become more common.
In summary, it may be convenient to criticize FOURIER as a failure of genetic medicine, as the composite cardiovascular endpoint was less than what many had hoped and the current drug price is high. However, don’t let those two critical issues muddle the importance of the trial itself and the impact on genetic-drive drug discovery.
What I have tried to do here is reminded readers of the bigger picture, at least as that picture pertains to genetically-driven drug discovery and development. The anti-PCSK9 / FOURIER story reinforces the concepts that human genetics can (1) identify novel drug targets that differentiate from standard of care; (2) speed pre-clinical development to test proof-of-concept in humans, but only after the painstaking task of unraveling human biology; (3) provide confidence in surrogate endpoints for regulatory approval, especially for biomarkers that are not routinely used in clinical care; and (4) provide guidance on dose-response curves, and therefore target dose for a large and expensive clinical trial.
Summary of news reports and blogs:
Matthew Herper Forbes blog, key points: (1) background and high-level summary; (2) Lower-than-expected results, but when broken down by specific outcomes (e.g., heart attack), findings were more in-line with predictions (e.g., observed 27% reduction in heart attacks); (3) mortality unaffected, but duration of trial design may not be sufficient to observe benefits; (4) patient vignettes that emphasize how the drug might be prescribed; (5) perspective of payers; and (6) value to patients.
David Grainger Forbes blog, key points: (1) background, trial design, and results; (2) magnitude of the reduction is disappointing (15% observed vs 25-30% predicted lower risk); (3) “stubborn rump” of CV events beyond LDL lowering; (4) mortality benefit likely seen over time, just not in this study of ~2 years duration; and (5) some disappointment because of potential for lower economic returns: “not because of any disappointment with their therapeutic profile, but because the treatment landscape into which they are being launched is very different from when development started and their cost is unavoidably high due to the frequent, large doses that are required for efficacy.”
Derek Lowe Science Translational Medicine blog, key points: (1) high-level background of genetics for drug R&D, including Mendelian diseases and GWAS; (2) importance of druggability, in this case a monoclonal antibody against the circulating PCSK9 protein; (3) value of CV risk reduction to payers; and (4) word of caution about genetic-based drug discovery.
Larry Husten Cardiobrief blog, key points: (1) general background; (2) FOURIER results, including primary and secondary endpoints; and (3) implications, as assessed by experts in the field, noting that “all agreed that that trial represents a remarkable and positive example of the rapid translation of genetic research to clinical therapeutics.”
Harlan Krumholz article on NPR.org, key points: (1) high-level overview of anti-PCSK9 therapy and FOURIER trial; (2) “the trial represents good, tangible evidence that the drug can reduce risk”; (3) “the hope that cardiovascular disease would be eliminated by these drugs is dimmed”; and (4) “study raises the issue of pricing and value”.
Nature N&V, key points: (1) high-level overview of anti-PCSK9 therapy and FOURIER; (2) “rough road” for PCSK9 therapies, including a drug (bococizumab) from Pfizer that was discontinue; and (3) value proposition: “The new results — from a trial with more than 27,500 participants — vindicate the concept that inhibiting PCSK9 can control cholesterol and heart-disease risk. The question now is whether physicians and health-care payers will consider that benefit great enough to warrant the annual price tag of roughly US$14,000.”
NEJM editorial, key points: (1) brief history of PCSK9 discovery and anti-PCSK9 therapies, including summary of pivotal clinical trials that led to FDA approvals in 2015; (2) brief summary of FOURIER results, including efficacy and safety findings; and (3) general implications for health care providers: “It is anticipated that the results of the FOURIER trial will soon be implemented in international guidelines regarding the treatment of high-risk patients, directing clinicians in the use of this new and expensive class of drugs.”