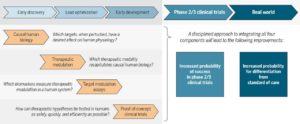
I know it may seem strange that I am commenting on my own commentary. While writing the Science Translational Medicine (STM) article (here, here), I wanted to focus on a clear vision for improving R&D productivity via integrating human biology, therapeutic modulation, pharmacodynamics biomarkers, and proof-of-concept clinical trials (see Figure 1 of the manuscript, also above). Too often, these four key concepts get lost amongst the many steps in drug development. I won’t revisit these concepts here, as I encourage you to read the article itself.
However, there is much more to R&D productivity than just these four concepts, and the purpose of this blog is to broaden the perspective piece a bit.
[Disclaimer: I am a Merck/MSD employee. The opinions I am expressing are my own and do not necessarily represent the position of my employer.]
1. Drug discovery should always start with the patient. Every drug discovery journey must begin with a clear definition of the unmet medical need. As a former practicing rheumatologist, I always try to keep in mind the questions that a patient would ask me: “Why did I develop this disease?” “Will I respond to this medication?” “What is my prognosis?” “Will I ever be cured?“
These questions are also important in the context of the translational medicine model proposed in the STM article. The “right target” is not just about causal human biology (defined as a target that, when perturbed, has the desired effect on human physiology). It is also about how the biology of the target fits into what is known about the current standard of care. For some diseases like Alzheimer’s disease, this is relatively straightforward, as there are no therapies that modify disease progression. For other disease, such as rheumatoid arthritis – my former clinical specialty – the unmet need is more specialized, as there are effective therapies that control disease symptoms.
2. Discoveries occur within a dynamic biomedical ecosystem. A casual reader of the STM article might assume that all components of the translational medicine model must reside within the walls of a single institution. Au contraire! For almost every approved drug, different components of the biomedical ecosystem – academics, small biotech companies, large pharmaceutical companies, government, foundations, venture capital finance – contributed in different ways.
As I have written about recently (link here), and others such as Bernard Munos have written about far more insightfully (here), the biomedical ecosystem is constantly evolving. What is confusing yet exhilarating, frustrating yet inspiring is that the ecosystem has many experiments underway to understand the best mix of these components. As an example, J&J is experimenting with a new open model of innovation (see recent Fortune magazine articles here, here). Many companies are moving to geographic regions such as Boston/Cambridge and the Bay Area, which have a high density of various components of a healthy ecosystem.
3. Innovation must occur in other areas of the drug development pipeline, too. While I highlighted the importance of discovery and early clinical development, there are other critical components of R&D productivity. I do believe strongly that innovation must continue to occur in late-stage clinical trials, the regulatory environment and in monitoring clinical outcomes in the real world. Fortunately, there are many new innovations – mobile devices, whole genome sequencing, electronic health records – occurring in these areas, too.
I know that there are many, many more components of the drug discovery process that impact R&D productivity…too many to do justice in this short blog. If you have comments on what you think is most important, please post comments below or tweet!