[I am an employee of BMS. The views expressed here are my own.]
The blog is long, so I will start with an executive summary. (You can download a pdf copy of the blog here.) Pharmacologic intervention has the opportunity to impact disease progression in the SARS-CoV-2 / COVID-19 crisis. Repurposing of approved therapies is the fastest way to impact patients today, as these medicines have regulatory approval to enable investigator-initiated trials and have a manufacturing process to ensure drug supply. Here, I focus on a specific clinical inflection point in COVID-19 disease progression – hospitalized patients early in their disease course and with signs of a maladaptive immune response, with the intervention intended to prevent disease progression and admission to the ICU. Based on an understanding of disease biology today – which is still quite limited – this clinical inflection point is due to a “maladaptive immune response” seen early in the disease course in patients who later progress to critical illness. Rigorous clinical trials are required to test therapeutic hypotheses related to repurposed therapies, which need to be done in a clinical setting caring for extremely sick patients. Finally, I describe additional research that is required to understand the biology of SARS-CoV-2 / COVID-19, and how such research (e.g., human genetics) that may help with future repurposing efforts while minimizing disruption of patient care.
Our society is at a critical juncture in the SARS-CoV-2 / COVID-19 pandemic. The number of confirmed cases is expanding at an alarming rate (here). There is legitimate concern that our health care system will soon be overtaken by patients requiring medical attention (here). Further, epidemiological models suggest that the pandemic will continue into 2021, and perhaps beyond depending availability of a vaccine or the acquisition of herd immunity (here). Accordingly, there has been an appropriate emphasis on diagnostic testing, health care infrastructure, and public health measures such as physical distancing and masks to “flatten the curve”. Indeed, non-pharmacological interventions are the most important means of controlling viral spread until a vaccine or the acquisition of herd immunity.
Pharmacological intervention can also flatten the curve of new infections. In the long term, new medicines that prevent infection (e.g., vaccines), neutralize circulating virus (e.g., antibodies directed against structural proteins [here]), or inhibit viral replication (e.g., direct-acting antivirals) will be developed and deployed. Unfortunately, this will likely take at least 12-18 months, as it takes time to demonstrate efficacy in clinical trials and to scale manufacturing to supply drug to populations.
An alternative approach – which could potentially be implemented today – is to repurpose existing therapeutics to intervene at key points in disease progression among those newly infected (here, here, here). There is early evidence that antivirals such as remdesivir – originally developed to fight Ebola virus – can reduce viral replication, which could be beneficial early in the course of SARS-CoV-2 infection. Therapies such as interferon beta – developed to treat multiple sclerosis – act by increasing expression and concentration of anti-inflammatory agents while downregulating expression of proinflammatory cytokines. Other therapies such as hydroxychloroquine – used to treat autoimmune diseases such systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) – may impair entry of the virus into cells, which could also be beneficial if given early in disease, although the results remain controversial (here). And anti-IL6 therapy – developed to treat autoimmune diseases such as RA and now also used to create cytokine release syndrome secondary to CAR-T therapy – is being tested as a therapy to treat high inflammatory states in severe cases of COVID-19 disease (here). To date, no repurposed therapy has completed an adequately powered randomized control trial (RCT) in COVID-19. A listing of ongoing trials can be found on the BioCentury coronavirus website (here).
It is first important to ask: at what point in COVID-19 disease progression should such repurposed therapies be tested? The answer depends on many factors: how and when patients are diagnosed; the ability to conduct a clinical study in a health care system in which providers are heroically caring for extremely sick patients; and the point in disease most likely to address the major unmet medical need. Most patients in the US, for example, are asymptomatic or have mild disease and therefore do not come to the hospital for treatment. While such a patient population might be ideal for a repurposed therapy to prevent progression to severe disease, these patients may be more difficult to enroll in a RCT.
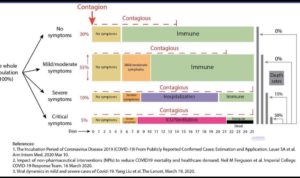
In this blog I focus on a specific point in disease progression – those with documented SARS-CoV-2 infection whose COVID-19 symptoms have progressed to the point where they require hospitalization but before the severe/critical stage requiring treatment in an intensive care unit (ICU). This is not only a sizeable portion of patients – approximately 15% by most reports (here; figure below adapted based on data from China) – but also represents a population of patients that, if appropriately managed, would address an important concern of our health care systems today: preventing the progression to critical disease requiring ICU care.
There are at least three challenges to this approach, which are introduced in this paragraph and then described in more detail below. First, the ability to rationally select which approved therapies will have the highest probability of success. This is a challenge because of incomplete data on SARS-CoV-2 / COVID-19 pathogenesis and the sheer number of therapeutic options. There are >2000 approved therapies, not to mention hundreds (if not more) therapies such as remdesivir that are still in clinical development. Second, the ability to test these therapies in a sufficiently rigorous clinical trial framework in the midst of a crisis. Any clinical trial will need to rely on clinical characteristics (e.g., increased respiratory rate, low oxygen saturation), lab abnormalities (e.g., increased IL6, ESR, CRP, ferritin, D-dimer, LDH) or imaging studies (e.g., chest x-ray for pneumonia, echocardiogram for myocarditis) that are collected as part of routine patient care. Similarly, any clinical trial protocol must be designed to give treating physicians on the frontlines sufficient flexibility to make decisions, as these physicians are carrying an enormous burden in caring for extremely sick patients under suboptimal conditions. And third, the ability to scale production and supply drug to the population in the event that a clinical trial is positive. There are reported shortages of repurposed therapies that have possible evidence of efficacy based on observational studies, and this may worsen as the pandemic continues.
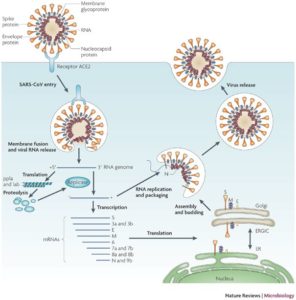
In order to repurpose therapies to prevent the progression to critical disease requiring ICU care, it is important to understand SARS-CoV-2 / COVID-19 viral life cycle and pathogenesis. The viral structural S (or spike) protein facilitates viral entry into host target cells. The S protein has a conserved receptor binding domain (RBD) and RB motif (RBM) that binds tightly to the angiotensin-converting enzyme 2 (ACE2) human receptor. Priming of the S protein is then achieved by the action of the human type II transmembrane serine protease, TMPRSS2, resulting in the fusion of viral and cellular membranes. Once inside of the cell, the virus releases its RNA into the cytoplasm of the infected human cell, which undergoes translation to produce the viral proteins required for subsequent viral RNA replication and transcription. Some of these proteins serve as the components of future virus particles (e.g., replication complex to make more RNA, structural proteins), while others suppress the immune response to promote viral replication and propagation. Viral protein and RNA components assemble in the Golgi to form new virons, which are released from the cell via a non-lytic mechanism. The SARS-CoV-2 life cycle is depicted below (based on SARS-CoV life cycle, see here).
SARS-CoV-2, unlike the closely related SARS-CoV, appears to be more efficient at human-to-human transmission, infecting ~2.5 people for every infected person. The high viral load during the early phase of infection might account for this enhanced transmissibility. SARS-CoV primarily infects epithelial cells within the lung. The virus can enter macrophages and dendritic cells but leads to an abortive infection. Nevertheless, these myeloid cells may be important in inducing the observed pro-inflammatory cytokines and contributing to disease progression. This may also be the case for SARS-CoV-2. The exact mechanism of lung injury and cause of severe disease is unknown in SARS-CoV-2 infected patients.
The innate and adaptive immune systems are important in clearing virus during the early stages of SARS-CoV-2 infection. What is provided in this paragraph is a general description of how the immune system fights viruses. There are many unknowns about how the immune system fights SARS-CoV-2, and so the specifics may differ during the progression in COVID-19. In general, the first line of defense against viral infection is an innate immune response triggered by pattern recognition receptors (PRRs) that recognize components of the virus, such as unique structural features found within the viral RNA genome. Stimulation of these PRRs activate intracellular signaling pathways that result in the release of type I interferons. This, in turn, leads to activation of the interferon receptor through the JAK/STAT pathway which up-regulates the transcription of a set of genes that orchestrate an anti-viral response. Activation of PRRs on antigen presenting cells leads to a series of cellular changes, such as up-regulation of co-stimulatory receptors, which enhances their ability to prime naïve T cells for the initiation of an adaptive immune response. Activated cytotoxic CD8 T cells kill virally infected cells. Activated CD4 helper cells promote a germinal center reaction that leads to the expansion of B cells that make high affinity antibodies that may neutralize the virus. Memory and B cells persist after clearance of the virus, which may protect against future infections.
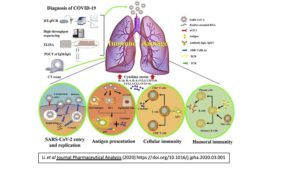
Figure from Li et al Journal Pharmaceutical Analysis (2020) https://doi.org/10.1016/j.jpha.2020.03.001
There is an emerging pattern of the immune response to SARS-CoV-2 infection (here). Early in COVID-19, there is a reduction in lymphocyte count. As disease progresses, there is an increase in inflammatory markers such as C reactive protein (CRP) and pro-inflammatory cytokines (IL-6, TNFα, IL-8). There is atrophy of the spleen and lymph nodes, along with reduced lymphocytes in lymphoid organs. Monocytes and macrophages predominate in the lungs, with minimal lymphocytes infiltration. In some patients, there are features of T cell exhaustion (here).
While the immune response is sufficient to clear the virus in most infected individuals without untoward damage to the host, a “maladaptive immune response” appears to emerge in some, leading to severe complications requiring ICU care (here, here). A failure to efficiently clear the virus early may result in an exaggerated and prolonged immune response which can manifest itself as a cytokine storm. This may be more likely to occur in immunocompromised patients but can also be seen in otherwise healthy individuals. Pro-inflammatory cytokines, such as IL-1, IL-6 and TNFa, distribute via systemic circulation, increasing blood flow and enabling leukocytes and plasma proteins to reach extravascular sites of injury. These responses may compromise organ function when tissue edema causes a rise in extravascular pressures and a reduction in tissue perfusion. Lung injury is a common consequence of a cytokine storm in the alveolar environment which can progress to acute respiratory distress syndrome (ARDS) and may also be seen with SARS-CoV-2. Compensatory repair processes initiated after the onset of inflammation can restore tissue function, but in some cases of severe inflammation, healing occurs with fibrosis, which can result in permanent organ dysfunction. This process is somewhat analogous to the way in which the immune system turns against the body in autoimmune diseases. Indeed, many therapies developed to treat autoimmune diseases are ideal candidates to prevent a maladaptive immune response.
Thus, the maladaptive immune response represents a key inflection point when a patient with mild-to-moderate symptoms progresses to severe disease requiring ICU care. As above, the reason this inflection point is important is that our health care system may not have the capacity to manage the projected surge in COVID-19 patients requiring ICU treatment. (It is important to note that ICU capacity varies from hospital to hospital, as well as by region in the US. Further, most US hospitals are adopting new measures to address the projected surge in critically ill COVID-19 patients.) This inflection point is important to prevent progression to potentially irreversible damage, morbidity, and mortality. Accordingly, therapies that prevent a maladaptive immune response have the potential to delay or prevent progression to severe disease requiring ICU care, and thereby prevent ICU overload which is threatening our health care system.

Understanding the time course from initial SARS-CoV-2 infection to a maladaptive immune response leading to severe COVID-19 disease is important in terms of thinking about categories of therapies for therapeutic intervention (see figure, above).
(1) A vaccine would work at the very beginning to prevent infection. A vaccine (and/or herd immunity) will be essential to recover from the pandemic. As vaccines need to be tailored to the virus itself, vaccines are not candidates for drug repurposing and will not be further discussed here.
(2) Passive immunotherapy (i.e., antibodies that bind to and neutralize circulating virus) may be used prophylactically in high-risk individuals to prevent infection or early in the infection process to prevent viral replication and spread (here). As with vaccines, these will not be discussed further in this blog, as the therapies need to be tailored to the virus.
(3) Direct-acting antivirals (i.e., bind to viral proteins to inhibit function) prevent viral replication and spread early in infection. While most antivirals need to be tailored to the virus, some (e.g., remdesivir) are being repurposed to fight SARS-CoV-2. While this is an important category of therapies early in disease, we will not consider it further here, as this does not address the turning point of a maladaptive immune response except indirectly by directly altering viral propagation.
(4) Indirect-acting antivirals may disrupt the viral life cycle (e.g., cell entry [ACE2 decoy, hydroxychloroquine], interferon suppression [interferon beta]) and therefore prevent viral replication and spread early in infection, as well as prevents progression to more severe disease. For example, SARS-CoV nucleocapsid (N) structural protein has been shown to inhibit IFN beta production by inhibiting TRIM25-mediated RIG-I ubiquitination and activation. The same process may be occurring with SARS-CoV-2. There will be opportunities for re-purposing in this category (e.g., hydroxychloroquine, interferon beta). While acting early in disease, these interventions may prevent progression to a maladaptive immune response.
(5) Anti-inflammatories that dampen a maladaptive immune response (e.g., hydroxychloroquine) to prevent a patient from progressing from mild/moderate to severe disease requiring ICU care. This category is the primary focus on this blog.
(6) Anti-inflammatories to treat cytokine storm (e.g., anti-IL6). There is overlap between the two anti-inflammatory categories. Still, it is important to separate them, as there may be opportunities for a therapy to work in one and not the other.
What is a pragmatic clinical trial design to test the therapeutic hypothesis that repurposed anti-inflammatory therapies can dampen a maladaptive immune response? Any clinical trial will need to rely on clinical characteristics and lab abnormalities that are collected as part of routine patient care. Similarly, any clinical trial protocol must be designed to give treating physicians on the frontlines sufficient flexibility to make decisions.
While it is probably self-evident, it is worth remembering that health care providers on the front lines are confronted with unprecedented challenges. They are caring for extremely sick patients with incomplete tools: there is an insufficient supply of personalized protective equipment (PPEs); there are too few diagnostic tests for acute infection and protective immunity; and there are not enough ventilators to care for predicted surge of patients.
Given these dire circumstances, the operations for any clinical trial must be extremely pragmatic – but at the same time sufficiently rigorous to draw firm conclusions. Inclusion criteria must be a standard part of patient care available in a timely matter. Similarly, any biomarkers and clinical outcome measures of safety and efficacy must also be standard of care. It is not realistic to implement bespoke features into a COVID-19 clinical trial.
Another consideration is the role of regulatory authorities in approving and monitoring the outcome of a clinical trial (here). Approved drugs can generally be prescribed for any purpose prescribers determine are appropriate based on their medical judgment. Under this scenario a master protocol – approved by an ethics board at an institution, hospital system or trial network (e.g., RECOVERY) – should be sufficient to launch an investigator-initiated trial (IIT). In some instances, an investigational new drug (IND) application may be required. For a drug still in clinical development (i.e., not yet approved by a regulatory authority), the situation is more complex. While it is possible that regulatory authorities may consider alternative approaches for SARS-CoV-2 / COVID-19, it is likely that most non-approved drugs will require regulatory input before initiating a clinical trial.
For a trial designed to prevent or delay patients with mild-to-moderate symptoms from progressing to the ICU, the following could be considered:
- Ensure appropriate consents are obtained from individuals, given the experimental nature of these potential treatments for this condition.
- Focus on mild-to-moderate symptomatic SARS-CoV-2 patients with documented infection, either before hospitalization when symptoms are mild (fever, cough, normal oxygen saturation) or following hospitalization but not yet requiring ICU care.
- For hospitalized patients, inclusion criteria include those who have early signs of a maladaptive immune response:
- Peripheral infiltrates on chest x-ray
- Moderate-to-severe symptoms (e.g., RR ≥30; O2 sat ≤93% room air)
- Evidence of inflammation (e.g., high IL-6, ferritin, LDH, ESR, CRP; low lymphocyte count)
- Start with an open label, observational trial at a small number of centers:
- ~15 patients on active vs 15 matched historical controls in same hospital
- Background of antiviral therapy and other SOC (as determined by local system)
- Primary outcome at 21-28 days: composite end point of mechanical ventilation, ICU admission, sepsis, organ failure, death
- Secondary outcomes – especially data captured via electronic medical records – include time to primary events (e.g., hospital discharge), normalization of laboratory values (e.g., lymphocyte count), clinical parameters (e.g., oxygen saturation), complications (e.g., myocardial dysfunction)
- Incorporate the option to expand based on initial 15 patients DSMB review to a larger, multi-center, randomized controlled trial (RCT).
- If successful, the approach could extend to patients with mild symptoms, and could even include patients not yet hospitalized.
- Ultimately, firm conclusions and introduction of repurposed medicines as standard of care in COVID-19 must be based not on small, observational trials but on appropriately powered randomized controlled trials.
For any treatment that dampens the immune response, it will be important to understand the impact on protective immunity against SARS-CoV-2. As above, our communities will only emerge from this pandemic once a vaccine is developed and/or a sufficient portion of the population – ~50-80% by most estimates – acquires herd immunity (here). Indeed, the concept of “immunology passports” is being considered by some countries (here). A theoretical consideration is that a therapy that dampens the maladaptive immune response may also impact an individual’s ability to acquire protective immunity. Accordingly, any clinical study that repurposes medicines to treat the maladaptive immune response in COVID-19 should study the impact of this intervention on protective immunity.
Any adequately powered trial with a positive outcome will require adequate capacity to scale manufacturing to meet demand. This should be straightforward for many approved therapies in active clinical use. Even then, it is important to understand inventory before initiating a clinical study. Supply is a greater concern for those approved drugs not in active clinical use, as well as drugs still in clinical development.
Assets in clinical development (i.e., not yet approved by regulatory authorities) also serve as an important opportunity for repurposing in SARS-CoV-2 / COVID-19. As described above, there are at least three challenges to test unapproved therapies: selecting dose and schedule for a safe and effective trial; role of regulatory authorities in approving and monitoring the clinical trial; and manufacturing capacity to ensure clinical supply in the event of a positive study. If there is an exceptionally strong scientific rationale, however, then it may be worthwhile to repurpose a therapy and address the three challenges above as needed. Importantly, there is less urgency for this category of opportunities, as it will take time to gain confidence in the therapeutic hypothesis, to work with regulatory authorities, and to ensure sufficient clinical supply.
There should be a coordinated effort across industry to help test molecules in a consistent set of pre-clinical models to help make the best possible decisions. There are a number of ongoing efforts aimed to coordinate repurposing (e.g., Gates Foundation). A number of trials have been initiated by groups willing to share data (e.g., RECOVERY trial). If a therapy looks promising in pre-clinical studies, then an academic-industry consortium could help prioritize opportunities to test in a coordinated manner. Indeed, many companies are working together to coordinate efforts (here), as highlighted recently by Sue Desmond-Hellman in her excellent WSJ editorial (here). This will ensure that the most promising assets are tested quickly, and – if successful – manufacturing is scaled to meet the demand in a timely fashion.
It is important to remember that for repurposing to be successful, we need to understand disease pathogenesis…and unfortunately, surprisingly little is known about the biology of SARS-CoV-2 / COVID-19. Given estimates that the pandemic will continue into 2021 (and potentially beyond – see here), our community will be in a better position to repurpose therapies in the future as we learn more biology today. As such, it is important to prioritize research efforts in a pragmatic manner, and that this should be done via an industry-wide, pre-competitive collaboration.
It is important to collect structured clinical phenotypes and quantitative traits generated as part of routine care, and these data can be federated across health care systems. Examples of important clinical phenotypes include patient demographics (e.g., age, gender, co-morbid conditions, smoking history, socioeconomic factors); disease severity; COVID-19 complications (e.g., ARDS, myocardial dysfunction); duration of hospitalization; concomitant medications; time to progression to clinical endpoints. Examples of quantitative traits include viral load over time; lymphocyte count; inflammatory markers (e.g., ESR, CRP, IL6 levels, ferritin); other laboratory values (e.g., PT/PTT, D-dimer, troponin, liver enzymes).
It is important to collect select data not part of routine care, as long as such sample collection will not interfere with clinical care. For example, it would be valuable to genotype subjects to enable genome-wide association studies (GWAS) on the clinical and quantitative traits above (here). Immune repertoire profiling and humoral immunity to SARS-CoV-2 are also important, especially to characterize antibodies that neutralize the virus. Unbiased RNA-sequence and proteomics profiling would enable a deeper understanding of the underlying pathophysiology of the maladaptive immune response. Some of these profiling efforts could be done on discarded blood samples (e.g., DNA extraction, GWAS genotyping, antibody responses), which would greatly facilitate sample collection without disrupting clinical care. Other studies (e.g., RNA and proteomic profiling) require specialized sample collection and may be more difficult to implement, especially given biosafety considerations of collection processes.
As data are collected, it is important to compare the output to other natural conditions of a maladaptive immune response, as this could help with repurposing. There are certain conditions such as macrophage activation syndrome and hemophagocytic lymphohistiocytosis that appear to resemble the maladaptive immune response in COVID-19. There are other forms of cytokine storm that may be different (e.g., CAR-T therapy-induced cytokine release syndrome). It will be important to compare immunological signatures from COVID-19 to other disease states to learn how to repurpose therapies. Another approach is to utilize population databases of patients with underlying immunological conditions who are on anti-inflammatory medicines (here). While this approach is complex due to confounders (e.g., long-standing treatment in a disease with underlying immune defects), it should be possible to determine if any therapies protect or exacerbate outcomes in COVID-19.
It will also be important to understand the key drivers of a maladaptive immune response. For example, LDH levels are associated with more severe disease (here), which could be a marker for something knows as pyroptosis, an inflammatory form of cell death (tweetorial here). Other studies have shown that T cell exhaustion may be correlated with severe disease, as increasing PD-1 and Tim-3 expression on T cells could be seen as patients progressed from prodromal to overtly symptomatic stages (here). These two mechanisms (pyroptosis, T cell exhaustion) point to different ways in which a maladaptive immune response could lead to cytokine storm, and in turn two different approaches to treat progression to severe COVID-19.
One final comment on research for repurposing: the role of human genetics. It is known that medicines whose targets are rooted in human genetics are more likely to demonstrate evidence of clinical efficacy (recent biorxiv preprint here). Equally important, human genetics can help guide indication selection, including the selection of new indications for established medicines. To date, few studies have look at the role of human genetics in predicting COVID-19 outcomes (here, here). If successful, this could be a potentially powerful approach to guide drug repurposing. Say, for example, that the CTLA4 variants that are associated with rheumatoid arthritis and other inflammatory diseases are also associated with disease progression in COVID-19. Such a finding would provide strong justification for an abatacept trial in COVID-19. There is a long list of immune therapies for which there are common genetic variants that could be used as genetic instruments in a COVID-19 repurposing effort: CTLA4 – abatacept; IL2RA – low-dose IL2 to boost Tregs; IL6R – tocilzumab, sarilumab; IL23A / IL12B / IL23R – ustekinumab; and IL18 – tadekinig alfa.
Conclusions
The COVID-19 pandemic represents one of the greatest public health crises of the last century. A vaccine and/or herd immunity will ultimately be required for the crisis to come to an end. Indeed, public health measures (e.g., physical distancing, hand washing, masks) are the most important interventions to flatten the curve today. It may be that pharmacological interventions can help in the near term, however. To dampen the maladaptive immune response that accompanies the progression to severe COVID-19 requiring ICU care, it may be possible repurpose existing anti-inflammatory therapies. An advantage of this approach is that such therapies are available for clinical testing now, and, if successful in appropriately powered RCT, can be manufactured to meet demand. Given what is known today, there are several reasonable therapeutic hypotheses that are being tested. Unfortunately, there are many unknowns about the immune response to SARS-CoV-2, which limits the utility of repurposing existing therapies. Nonetheless, the magnitude of the current COVID-19 crisis and the potential for helping patients argues for well-designed RCT to test therapeutic hypotheses related to the maladaptive immune response.

