We held our second annual GpGx retreat last week at the Dolce Norwalk Conference Center in Norwalk, CT. The theme was “integrate and elevate”: integrate across Translational Medicine and elevate our mission to infuse cutting-edge genetics and genomics into Merck’s pipeline. What follows is a brief recount of the event.
For those who don’t like looking at photos of someone else’s family vacation, this blog post might not be for you. However, for those curious about life within pharma – read on! You might be surprised that the basic principles that create a strong community within academics or a small biotechnology company are at play within a large company like Merck. I also provide examples of Translational Medicine in action: picking the right target based on causal human biology; developing the right biomarkers based on mechanistic insight of the target; selecting therapeutic molecules (e.g., biologics) in a modality independent manner; and testing clinical proof-of-concept (POC) in small Phase Ib/IIa clinical studies.
[Disclaimer: I am a Merck/MSD employee. The opinions I am expressing are my own and do not necessarily represent the position of my employer.]
At the start of the retreat, I provided an overview on our theme: “integrate and elevate” (see slides here). This topic was chosen as (a) our GpGx team was recently integrated with Translational Biomarkers and Translational Pharmacology to create a new Translational Medicine Department, and (b) our first year was successful, but we have opportunities for continuous improvement. A recent example of “elevation” was our recent pre-competitive collaboration with Finland, the Broad Institute, and three other companies around genetic biobanks (see here).
Manu Chakravarthy gave a presentation entitled “Rethinking the beginning: anchoring target validation in human biology”. Manu emphasized the theme of causal human biology to select new drug targets. He gave examples beyond human genetics, citing examples of pharmacological repurposing and longitudinal profiling of human subjects. Further, he provided a description of the range of experimental medicine tools that can be used to identify and validate new drug targets in the ideal model organism, humans.
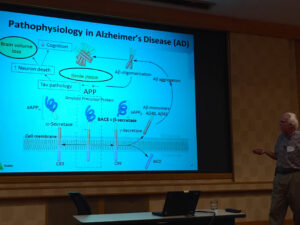
Next, Jeff Evelhoch discussed “How Translational Biomarkers Impact the Pipeline”. Jeff leads a team of over 100 scientists who discovery, qualify and deploy biomarkers across Merck’s pipeline. He provided examples of clinical and imaging biomarkers from our studies in Alzheimer’s disease, including those used to test proof-of-mechanism in our clinical trials.
Traveling all the way from Palo Alto, CA, Terri McClanahan delivered a talk “Harnessing Translational Approaches to Power Biologics Development: Focus on Immuno-Oncology”. As I have commented elsewhere (see here), the field of immunotherapy is revolutionizing cancer treatment. Terri’s presentation reminded everyone that Merck is at the forefront of discovery research and clinical implementation in this exciting area of human biology. She also discussed the steps to develop a new biologic, as shown in the image below.
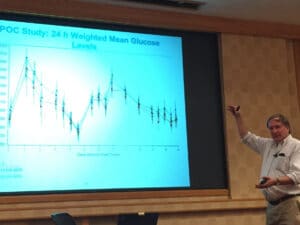
The ultimate test of a therapeutic hypothesis is to take a molecule into humans, and Gene Marcantonio described how this is done in an early proof-of-concept (POC) study: “Using the SCD-1 program as an example for developing a POC strategy”. Gene highlighted the practical aspects of getting a molecule into humans, and how important it is to work closely with colleagues in preclinical development (e.g., safety, pharmacokinetics and drug metabolism, formulations). He also explained the importance of clinical readouts such 24-hour weighted mean glucose levels (see image below).
To conclude the scientific presentations on the first day, Marian Iwamoto delivered a presentation on how all of this comes together to advance a new therapeutic into the clinic: “Clinical Development Program of Integrase Inhibitors” (see image below). One of my favorite parts of her talk was when she described how Merck scientists, led by Daria Hazuda, removed a key bottleneck in the development of this drug class to treat HIV. Specifically, Daria’s team developed the assays required to measure in vitro integrase activity. I like to use this as one of the many examples of “innovation” when scientists outside of industry criticize the lack of innovation in big pharma.
The next day was our Keynote address by Amy Espeseth, “Progress towards RNA vaccines for active and passive immunization”. She described the exciting work Merck is doing in collaboration with Moderna Therapeutics. Unfortunately, we don’t have a photo from Amy’s talk…but it was great!
The GpGx retreat went beyond scientific presentations, as we had a variety of social and team-building activities. The beautiful setting lent itself to relaxing lunches and ample time for social Browning motion (see photos below). We also subjected ourselves to a modern-day scavenger hunt, run by the company The Go Game.
All-in-all, it was a great chance for us to celebrate our numerous successes over the past year.